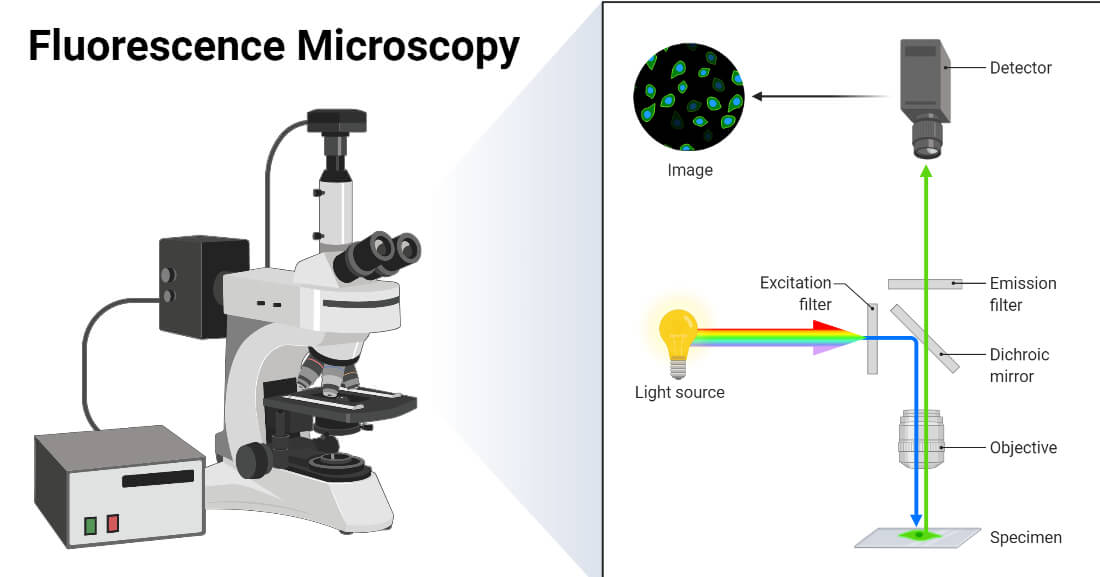
Light microscopes
Confocal laser scanning microscopy
Confocal microscopy uses a laser to illuminate a single point within the specimen and employs a pinhole in front of the detector to block out-of-focus light, enhancing optical sectioning and contrast. By scanning the laser across the sample in a raster pattern, confocal microscopy can generate high-resolution images of thin optical sections, allowing for three-dimensional reconstruction of the specimen. This technique is widely used in biological and medical research for imaging fixed and live samples, particularly where high resolution and optical sectioning are required (Pawley, 2006; Lichtman and Conchello, 2005).
| Versatility: |
Confocal microscopy is highly versatile and can be adapted for a wide range of biological imaging applications, including live-cell imaging, fixed tissue imaging, and various advanced techniques like fluorescence resonance energy transfer (FRET) and fluorescence recovery after photobleaching (FRAP). |
| XY Resolution: | Provides moderate lateral resolution (~200 nm) due to the elimination of out-of-focus light using a pinhole aperture, though it is still limited by the diffraction of light (Lichtman and Conchello, 2005; Pawley, 2006). |
| Z Resolution: | Offers improved axial resolution over widefield microscopy by optically sectioning samples, but resolution is limited by the size of the pinhole and diffraction effects (Pawley, 2006; Lichtman and Conchello, 2005). |
| Thick Sample Compatibility: | Suitable for imaging moderately thick specimens, but performance declines due to photobleaching and reduced penetration depth (Lichtman and Conchello, 2005). |
| Live-Cell Friendliness: | Confocal microscopy is suitable for live-cell imaging when laser power and exposure time are carefully controlled to minimize phototoxicity (Pawley, 2006). |
| Image Fidelity: | Produces high-fidelity images with good contrast by rejecting out-of-focus light, though photobleaching can reduce signal over time (Pawley, 2006; Lichtman and Conchello, 2005). |
| Multicolor: | Supports multicolor imaging with minimal spectral overlap, making it ideal for complex imaging studies (Pawley, 2006). |
| Temporal Resolution: | Offers moderate temporal resolution due to the time required for point-by-point scanning (Pawley, 2006). |
| Availability: | Widely available in research settings with numerous commercial options (Pawley, 2006). |
Confocal Spinning Disk Microscopy
Spinning disk confocal microscopy uses a disk with multiple pinholes that rotate rapidly, allowing simultaneous multi-point illumination and detection. This design enables faster imaging with lower phototoxicity and photobleaching compared to traditional point-scanning confocal microscopy. Spinning disk confocal is particularly useful for live-cell imaging and capturing dynamic processes in real-time (Nakano, 2002; Graf et al., 2005).
| Versatility: | Similar versatility to point-scanning confocal but with enhanced speed and reduced phototoxicity, making it better suited for dynamic live-cell studies (Nakano, 2002). |
| XY Resolution: | Comparable lateral resolution to point-scanning confocal, although slight blurring can occur due to light scatter from the disk (Nakano, 2002; Graf et al., 2005). |
| Z Resolution: | Similar axial resolution to point-scanning confocal but can be slightly lower due to overlapping pinholes (Nakano, 2002; Graf et al., 2005). |
| Thick Sample Compatibility: | Better suited for thicker samples than point-scanning confocal due to reduced photobleaching and faster acquisition speeds (Nakano, 2002). |
| Live-Cell Friendliness: | Highly suitable for live-cell imaging due to rapid image acquisition and lower phototoxicity (Graf et al., 2005). |
| Image Fidelity: | High image fidelity, though slightly lower than single-point confocal due to potential non-uniform illumination and light scatter from the spinning disk (Nakano, 2002). |
| Multicolor: | Excellent for multicolor imaging with appropriate filter sets, minimizing spectral crosstalk (Nakano, 2002). |
| Temporal Resolution: | Provides high temporal resolution due to rapid simultaneous multi-point scanning, ideal for capturing fast cellular dynamics (Nakano, 2002; Graf et al., 2005). |
| Availability: | Widely available but requires more specific equipment than point-scanning confocal (Nakano, 2002). |
Multiphoton Microscopy
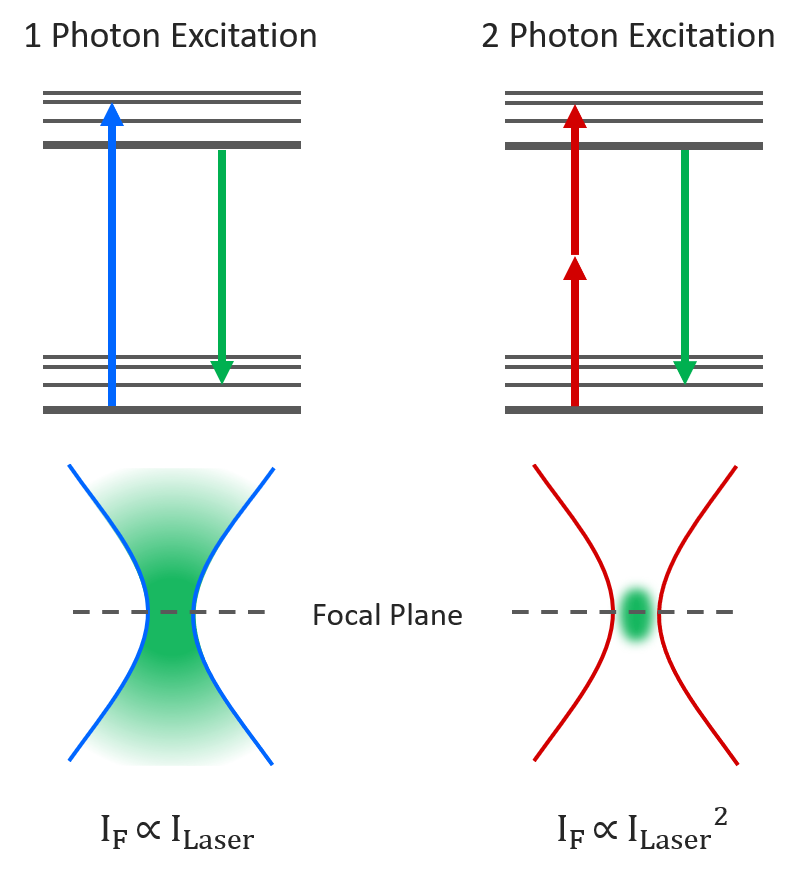
Multiphoton microscopy utilizes near-infrared laser light to excite fluorophores through the simultaneous absorption of two or more photons, which occurs only at the focal point. This method restricts excitation to the focal plane, minimizing photobleaching and phototoxicity outside the imaging plane. It is particularly well-suited for deep tissue imaging in live animals and thick specimens (Denk et al., 1990; Helmchen and Denk, 2005).
| Versatility: | Less versatile than other forms of microscopy due to specific requirements for fluorophores and longer excitation wavelengths. However, it excels in deep tissue imaging and live-animal studies (Denk et al., 1990). |
| XY Resolution: | Lower lateral resolution than confocal microscopy due to the use of longer excitation wavelengths, which decreases resolution but allows deeper tissue penetration (Zipfel et al., 2003; Helmchen and Denk, 2005). |
| Z Resolution: | Provides excellent axial resolution for thick tissues, thanks to localized excitation at the focal point and reduced out-of-focus fluorescence (Denk et al., 1990; Helmchen and Denk, 2005). |
| Thick Sample Compatibility: | Highly compatible with thick samples due to its ability to penetrate deep into tissues with minimal scattering and photodamage, making it ideal for in vivo imaging (Denk et al., 1990). |
| Live-Cell Friendliness: | Suitable for live-cell imaging in thick tissues, though requires careful control of laser power to avoid photodamage (Helmchen and Denk, 2005). |
| Image Fidelity: | High fidelity in thick specimens due to minimal background noise and reduced scattering outside the focal plane (Denk et al., 1990; Helmchen and Denk, 2005). |
| Multicolor: | Capable of multicolor imaging but limited by the range of available excitation wavelengths and the need for specific fluorophores (Zipfel et al., 2003). |
| Temporal Resolution: | Slower temporal resolution compared to widefield or spinning disk confocal due to the sequential scanning required for multiphoton excitation (Helmchen and Denk, 2005). |
| Availability: | Less commonly available than other light microscopy techniques due to higher cost and specialized equipment (Denk et al., 1990; Helmchen and Denk, 2005). |
Wide-field Microscopy
Widefield microscopy involves illuminating the entire specimen and capturing fluorescence from all focal planes. This technique provides rapid imaging and is particularly suited for thin specimens and high-throughput applications. However, it suffers from lower resolution and contrast for thick specimens due to significant out-of-focus light (Diaspro et al., 2005).
| Versatility: | Highly versatile for imaging a wide range of samples, particularly thin or monolayer specimens. It is commonly used in cell biology, histology, and high-throughput screening (Diaspro et al., 2005). |
| XY Resolution: | Limited lateral resolution due to the inclusion of out-of-focus light, which can cause blurring and reduce image clarity, especially in thicker samples (Diaspro et al., 2005). |
| Z Resolution: | Poor axial resolution because it captures all emitted light, including out-of-focus fluorescence, leading to significant loss of contrast and detail in the Z-axis (Diaspro et al., 2005). |
| Thick Sample Compatibility: | Not suitable for thick specimens due to the substantial accumulation of out-of-focus light, which severely degrades image quality (Diaspro et al., 2005). |
| Live-Cell Friendliness: | Highly suitable for live-cell imaging, particularly for thin specimens, due to its rapid imaging capabilities and low phototoxicity (Diaspro et al., 2005). |
| Image Fidelity: | Moderate image fidelity due to the presence of out-of-focus light, which reduces contrast and clarity in thicker samples (Diaspro et al., 2005). |
|
Multicolor: |
Effective for multicolor imaging but can suffer from cross-talk between channels, especially in thicker samples (Diaspro et al., 2005). |
| Temporal Resolution: | Provides excellent temporal resolution because it captures the entire field of view in a single exposure, making it ideal for fast imaging applications (Diaspro et al., 2005). |
| Availability: | The most accessible and widely available type of fluorescence microscope, with basic systems being affordable and easy to use (Diaspro et al., 2005). |
Light-Sheet Microscopy
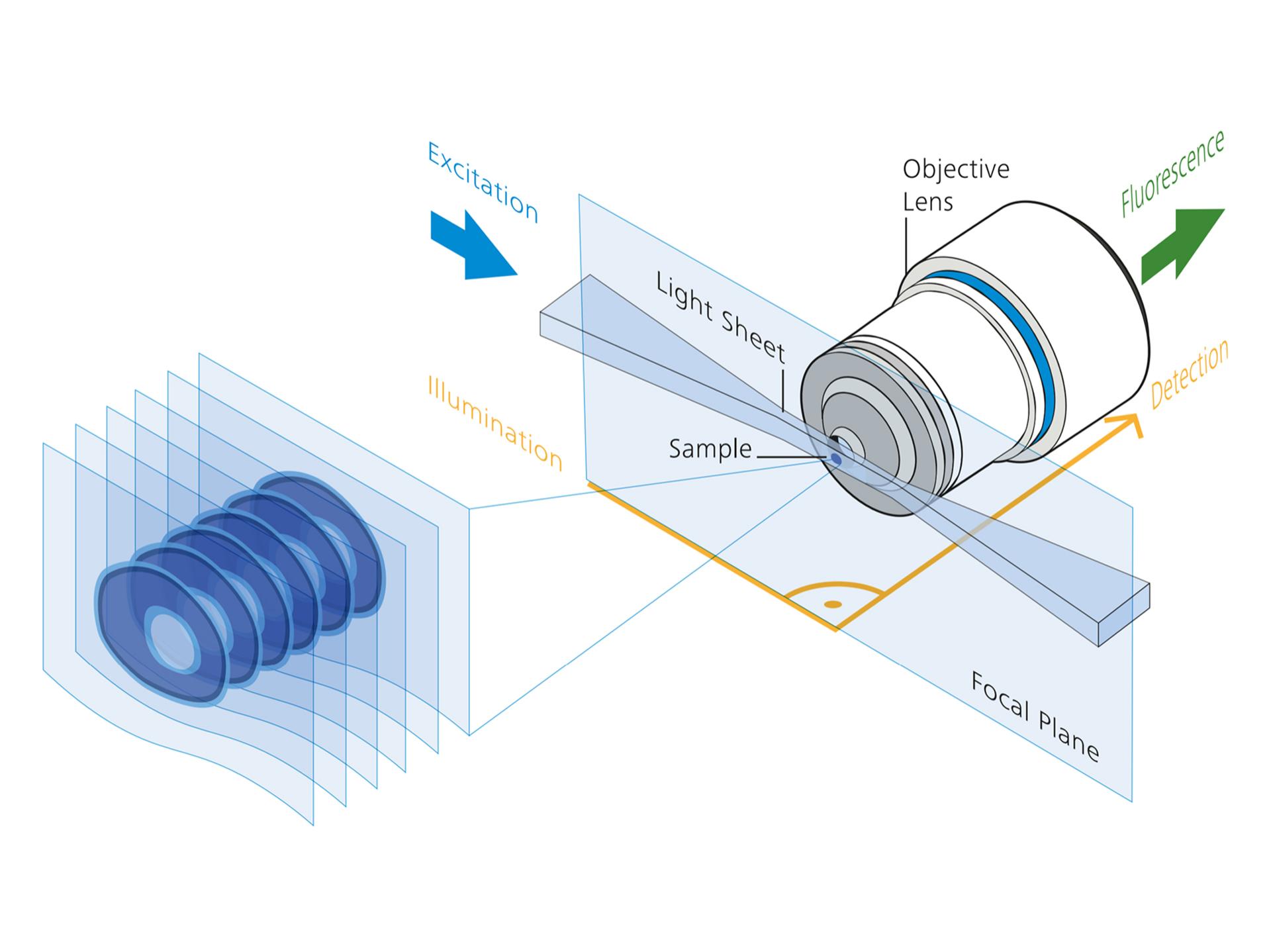
Light-Sheet Fluorescence Microscopy (LSFM) uses a thin sheet of laser light to illuminate a single optical plane of the specimen while an orthogonal detection system captures fluorescence from the illuminated plane. This method allows fast, high-resolution imaging of large, live specimens with minimal phototoxicity, making it ideal for developmental biology and neuroscience applications (Huisken and Stainier, 2009; Power and Huisken, 2017).
| Versatility: | Extremely versatile, allowing rapid 3D imaging of large samples such as embryos or entire organs. It is highly suitable for live imaging and developmental studies (Huisken and Stainier, 2009). |
| XY Resolution: | Provides high lateral resolution due to the thin plane of illumination and orthogonal detection, enabling fine detail imaging across large fields of view (Siedentopf and Zsigmondy, 1902; Huisken and Stainier, 2009). |
| Z Resolution: | High axial resolution is achieved by selective plane illumination and orthogonal detection, allowing for sharp sectioning and minimal background from out-of-focus light (Huisken and Stainier, 2009; Power and Huisken, 2017). |
| Thick Sample Compatibility: | Highly compatible with thick samples due to the thin light sheet that selectively illuminates one plane at a time, minimizing photobleaching and photodamage (Huisken and Stainier, 2009). |
| Live-Cell Friendliness: | Superior for live-cell imaging, enabling long-term observations with minimal phototoxicity and bleaching due to selective plane illumination (Huisken and Stainier, 2009; Power and Huisken, 2017). |
| Image Fidelity: | High image fidelity with minimized photodamage and effective optical sectioning, making it ideal for 3D imaging of large samples (Huisken and Stainier, 2009). |
| Multicolor: | Effective for multicolor imaging with reduced phototoxicity, suitable for observing multiple fluorophores in live specimens (Huisken and Stainier, 2009; Power and Huisken, 2017). |
| Temporal Resolution: | Superior temporal resolution due to fast plane illumination and orthogonal detection, ideal for dynamic live-cell imaging and real-time 3D reconstructions (Huisken and Stainier, 2009; Power and Huisken, 2017). |
| Availability: | Increasingly available in specialized research settings, but less common due to equipment complexity and cost (Huisken and Stainier, 2009). |
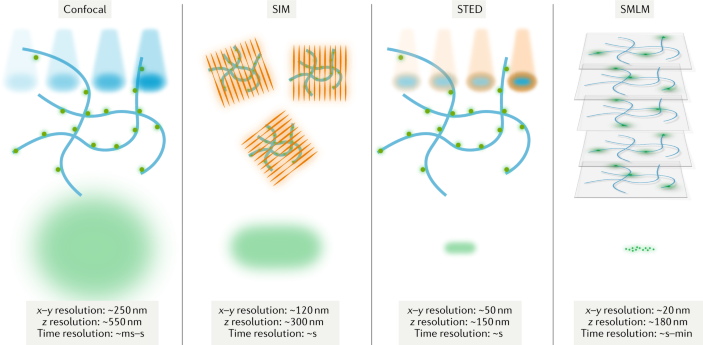
Super-Resolution Microscopy
Super-Resolution Microscopy techniques, including Structured Illumination Microscopy (SIM), Stimulated Emission Depletion Microscopy (STED), and Single-Molecule Localization Microscopy (SMLM, such as PALM and STORM), surpass the diffraction limit of conventional light microscopy, allowing imaging with nanometer-scale resolution.
| Versatility: | Super-resolution techniques are highly versatile, applicable to various biological questions requiring high spatial resolution, such as protein localization and molecular interactions. However, each method has specific sample preparation and imaging requirements (Schermelleh et al., 2019). |
| XY Resolution: | Provides exceptional lateral resolution well below the diffraction limit (~10-50 nm), depending on the technique used, making it ideal for studying subcellular structures (Hell and Wichmann, 1994; Betzig et al., 2006). |
| Z Resolution: | Enhanced axial resolution, particularly in 3D versions of SMLM and STED, though it can be limited by photobleaching and the need for specific imaging conditions (Huang et al., 2008; Harke et al., 2008). |
| Thick Sample Compatibility: | Super-resolution microscopy is less compatible with thick samples due to increased light scattering and potential photobleaching. However, specialized setups like light-sheet combined with super-resolution are being developed (Liu et al., 2015). |
| Live-Cell Friendliness: | While SIM is more suitable for live-cell imaging due to lower phototoxicity, STED and SMLM are generally less compatible due to high laser powers and long acquisition times (Gustafsson, 2000; Schermelleh et al., 2019). |
| Image Fidelity: | Provides high-fidelity images with nanometer-scale resolution, allowing detailed visualization of molecular structures and protein interactions (Schermelleh et al., 2019). |
| Multicolor: | Effective for multicolor imaging, especially with SMLM and STED, but care must be taken to minimize spectral overlap and photobleaching (Donnert et al., 2007; Rust et al., 2006). |
| Temporal Resolution: | Moderate temporal resolution; SIM provides faster imaging than SMLM and STED, which are slower due to the need for sequential imaging and depletion steps (Schermelleh et al., 2019). |
| Availability: | Increasingly available in specialized research facilities but requires advanced equipment and significant expertise, limiting widespread use (Schermelleh et al., 2019). |
References
- Beck, M., & Baumeister, W. (2016). Cryo-Electron Tomography: Can It Reveal the Molecular Sociology of Cells in Atomic Detail? Trends in Cell Biology, 26(11), 825-837.
- Bozzola, J. J., & Russell, L. D. (1999). Electron Microscopy: Principles and Techniques for Biologists. Jones and Bartlett Publishers.
- Denk, W., & Horstmann, H. (2004). Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biology, 2(11), e329.
- Denk, W., Strickler, J. H., & Webb, W. W. (1990). Two-photon laser scanning fluorescence microscopy. Science, 248(4951), 73-76.
- Diaspro, A., Bianchini, P., Vicidomini, G., Faretta, M., Ramoino, P., & Usai, C. (2005). Multi-photon excitation microscopy. Biomedical Engineering Online, 4(1), 36.
- Dubochet, J., Adrian, M., Chang, J. J., Homo, J. C., Lepault, J., McDowall, A. W., & Schultz, P. (1988). Cryo-electron microscopy of vitrified specimens. Quarterly Reviews of Biophysics, 21(2), 129-228.
- Egerton, R. F. (2016). Physical Principles of Electron Microscopy: An Introduction to TEM, SEM, and AEM. Springer.
- Fernández, J. J., Li, S., & Crowther, R. A. (2017). CTF determination and correction in electron cryotomography. Ultramicroscopy, 169, 72-82.
- Frank, J. (2006). Three-Dimensional Electron Microscopy of Macromolecular Assemblies: Visualization of Biological Molecules in Their Native State. Oxford University Press.
- Friel, J. J., & Lyman, C. E. (2006). X-ray mapping in electron-beam instruments. Microscopy and Microanalysis, 12(1), 2-25.
- Goldstein, J., Newbury, D. E., Joy, D., Lyman, C., Echlin, P., Lifshin, E., Sawyer, L., & Michael, J. (2017). Scanning Electron Microscopy and X-ray Microanalysis. Springer.
- Graf, R., Rietdorf, J., & Zimmermann, T. (2005). Live cell spinning disk microscopy. Microscopy Techniques, 95-114.
- Hayat, M. A. (2000). Principles and Techniques of Electron Microscopy: Biological Applications. Cambridge University Press.
- Helmchen, F., & Denk, W. (2005). Deep tissue two-photon microscopy. Nature Methods, 2(12), 932-940.
- Huisken, J., & Stainier, D. Y. R. (2009). Even fluorescence excitation by multidirectional selective plane illumination microscopy (mSPIM). Optics Letters, 32(17), 2608-2610.
- Kühlbrandt, W. (2014). Cryo-EM enters a new era. eLife, 3, e03678.
- Lichtman, J. W., & Conchello, J. A. (2005). Fluorescence microscopy. Nature Methods, 2(12), 910-919.
- Micheva, K. D., & Smith, S. J. (2007). Array tomography: A new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron, 55(1), 25-36.
- Nakano, A. (2002). Spinning-disk confocal microscopy—a cutting-edge tool for imaging of membrane traffic. Cell Structure and Function, 27(5), 349-355.
- Nogales, E., & Scheres, S. H. W. (2015). Cryo-EM: A unique tool for the visualization of macromolecular complexes in their native environment. Nature Methods, 12(1), 24-28.
- Pawley, J. B. (2006). Handbook of Biological Confocal Microscopy. Springer.
- Pawley, J. B. (1995). Fundamental limits in confocal microscopy. Springer.
- Peddie, C. J., & Collinson, L. M. (2014). Exploring the third dimension: Volume electron microscopy comes of age. Micron, 61, 9-19.
- Pennycook, S. J., & Nellist, P. D. (2011). Scanning Transmission Electron Microscopy: Imaging and Analysis. Springer.
- Power, R. M., & Huisken, J. (2017). A guide to light-sheet fluorescence microscopy for multiscale imaging. Nature Methods, 14(4), 360-373.
- Schertel, A., Snaidero, N., Han, H. M., Ruhwedel, T., Laue, M., Grabenbauer, M., & Simons, M. (2013). Cryo FIB-SEM: Volume imaging of cellular ultrastructure in native frozen specimens. Journal of Structural Biology, 184(2), 355-360.
- Siedentopf, H., & Zsigmondy, R. (1902). Über Sichtbarmachung und Größenbestimmung ultramikroskopischer Teilchen, mit besonderer Anwendung auf Goldrubingläser. Annalen der Physik, 315(1), 1-39.
- van Aken, P. A., & Liebscher, B. (2002). Quantification of ferrous/ferric ratios in minerals: New evaluation schemes of Fe L2,3 electron energy-loss near-edge spectra. Physics and Chemistry of Minerals, 29(3), 188-200.
- Williams, D. B., & Carter, C. B. (2009). Transmission Electron Microscopy: A Textbook for Materials Science. Springer.
- Zipfel, W. R., Williams, R. M., & Webb, W. W. (2003). Nonlinear magic: Multiphoton microscopy in the biosciences. Nature Biotechnology, 21(11), 1369-1377.
- Betzig, E., Patterson, G. H., Sougrat, R., Lindwasser, O. W., Olenych, S., Bonifacino, J. S., ... & Hess, H. F. (2006). Imaging intracellular fluorescent proteins at nanometer resolution. Science, 313(5793), 1642-1645.
- Diaspro, A., Bianchini, P., Vicidomini, G., Faretta, M., Ramoino, P., & Usai, C. (2005). Multi-photon excitation microscopy. Biomedical Engineering Online, 4(1), 36.
- Donnert, G., Keller, J., Medda, R., Andrei, M. A., Rizzoli, S. O., Lührmann, R., ... & Hell, S. W. (2007). Macromolecular-scale resolution in biological fluorescence microscopy. Proceedings of the National Academy of Sciences, 104(6), 2223-2228.
- Gustafsson, M. G. (2000). Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. Journal of Microscopy, 198(2), 82-87.
- Harke, B., Ullal, C. K., Keller, J., & Hell, S. W. (2008). Three-dimensional nanoscopy of colloidal crystals. Nano Letters, 8(5), 1309-1313.
- Hell, S. W., & Wichmann, J. (1994). Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Optics Letters, 19(11), 780-782.
- Helmchen, F., & Denk, W. (2005). Deep tissue two-photon microscopy. Nature Methods, 2(12), 932-940.
- Huang, B., Wang, W., Bates, M., & Zhuang, X. (2008). Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science, 319(5864), 810-813.
- Huisken, J., & Stainier, D. Y. R. (2009). Even fluorescence excitation by multidirectional selective plane illumination microscopy (mSPIM). Optics Letters, 32(17), 2608-2610.
- Liu, Z., Lavis, L. D., & Betzig, E. (2015). Imaging live-cell dynamics and structure at the single-molecule level. Molecular Cell, 58(4), 644-659.
- Nakano, A. (2002). Spinning-disk confocal microscopy—a cutting-edge tool for imaging of membrane traffic. Cell Structure and Function, 27(5), 349-355.
- Pawley, J. B. (2006). Handbook of Biological Confocal Microscopy. Springer.
- Rust, M. J., Bates, M., & Zhuang, X. (2006). Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nature Methods, 3(10), 793-795.
- Schermelleh, L., Ferrand, A., Huser, T., Eggeling, C., Sauer, M., Biehlmaier, O., & Drummen, G. P. (2019). Super-resolution microscopy demystified. Nature Cell Biology, 21(1), 72-84.
- Siedentopf, H., & Zsigmondy, R. (1902). Über Sichtbarmachung und Größenbestimmung ultramikroskopischer Teilchen, mit besonderer Anwendung auf Goldrubingläser. Annalen der Physik, 315(1), 1-39.
- Willig, K. I., Rizzoli, S. O., Westphal, V., Jahn, R., & Hell, S. W. (2006). STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature, 440(7086), 935-939.
- Zipfel, W. R., Williams, R. M., & Webb, W. W. (2003). Nonlinear magic: Multiphoton microscopy in the biosciences. Nature Biotechnology, 21(11), 1369-1377.